How copper influences the flavour of whisky
Copper, it’s all about the shinny shinny copper
In Part 2: Whisky stills, is it about the size, or how you use it? We looked at how the size and shape of a still as well as how it’s used can all affect the character of the spirit it produces. This time we’ll be talking about how the material the still is made from (in this case copper) effects the spirit it produces.
The beating heart of any distillery and one of its best photo ops is its still(s), a bit like the iron throne, they are often great gleaning metallic towers rising as high as the ceiling. Although thousands of shapes and sizes are used by different distilleries the world over there is one constant, the unmistakable old-worldly hue of copper.
It’s not hard to figure out why copper was originally chosen for the construction of metallic stills hundreds of years ago. It’s highly malleable, able to be bent and hammered into many shapes and curves, meaning it needs less joins, rivets, & plates – the weakest spot in any pressure vessel. Copper is also hard-wearing, easy to clean, cheap, abundant, & has great thermal conductivity meaning quicker heating and cooling. Despite all these benefits, it’s actually the chemistry of copper that makes it ideal for producing great whisky.
Many cultures and many old wives’ tales talk of the health benefits of drinking water from copper cups or cooking in copper pots and it has well documented anti-bacterial and anti-fungal qualities. It’s ability to catalyse sulphur-containing compounds is what matters to distillers though.
So what does copper actually do then?
Distilling isn’t a process of making something new, rather it’s a process of refining what’s already there, selectively letting some parts through while stopping others. And whereas the fermentation of barley produces many desirable flavours it also produces some undesirable ones. Most notably, volatile sulphur compounds including dimethyl-sulphides, trimethyl-sulphides, thiols, & mercaptans. Which combined are responsible for meaty, burnt egg, and ‘struck match’ aromas and flavours.
Managing the levels of these compounds in newly distilled spirit is one of the most important roles for a distiller, trace amounts are desirable and add body and depth, but too much and you’re left with an unpleasant spirit. Copper acts as a catalyst for these volatile sulphur compounds, converting them into shiny blue copper salt crystals that precipitate out of the spirit. Higher reflux levels will enhance copper contact increasing this catalytic effect, promoting fruity, estery, clean spirit, while preventing the development of unpleasant feinty aromas in the heart cut. Copper is also believed to promote the development of fruity esters through interactions with thermal energy and residual yeast cells in the wash. These effects result in the creation of a lighter, fruitier & more estery spirit.
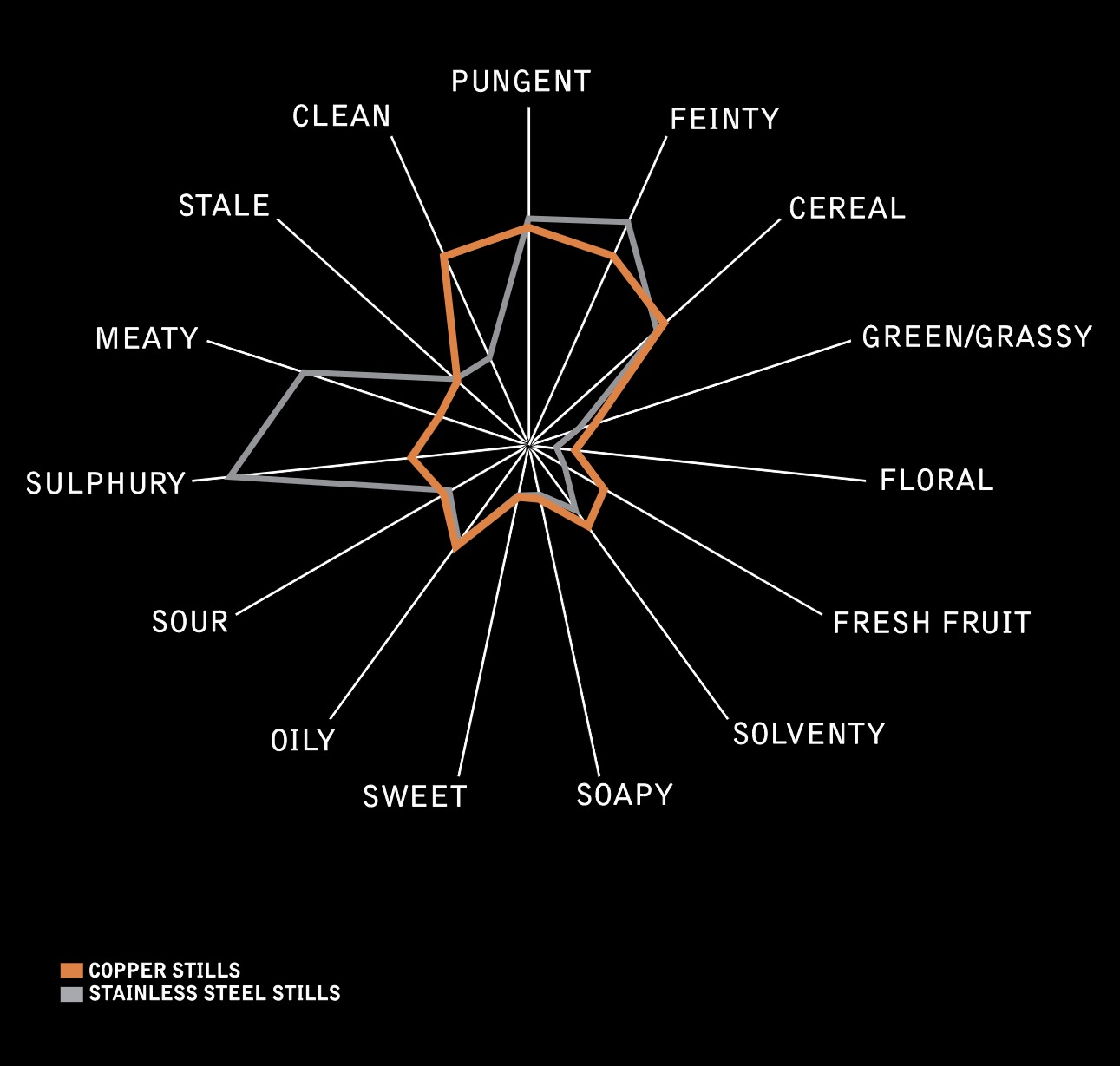
We used a lot of long words, so here’s a picture
These effects are highlighted in the diagram below which has been adapted from an article in the JOURNAL OF THE INSTITUTE OF BREWING VOL. 117, NO. 1, 2011 pp 109 (worth looking up if you’re interested in delving into some more copper whisky chemistry). It compares spirit distilled in both copper and stainless steel stills with a marked reduction in Sulphury, meaty and feinty notes clearly present when copper is used.
Copper is much more than just a pretty face or a throwback to simpler times, it’s an essential component in producing good whisky. Although we’ll admit, it makes a pretty good photo op 🙂
If you’re keen to learn more check out Part 4: Making whisky is all about the barrel right?
